

THE CLINICAL REPORT
A clinical report is automatically generated for every cancer patient at the end of the test. In the clinical report the following informations are available. Patient ID, the grade of general drug sensitivity of the tumor cells as an action of the different drugs killing events, specific informations and the therapy recommendations.
The report designed to help the hematologist or oncologist to choose the right therapy for the patient where patients’ own tumor cells have been exposed to the different drugs in vitro.
It must be emphasized that the final therapy should be decided by the physician in accordance with the patient specific data, disease and health status in all complexity. The clinical report should always only be considered as a therapy guidance.

THE KILLING EFFICIENCY
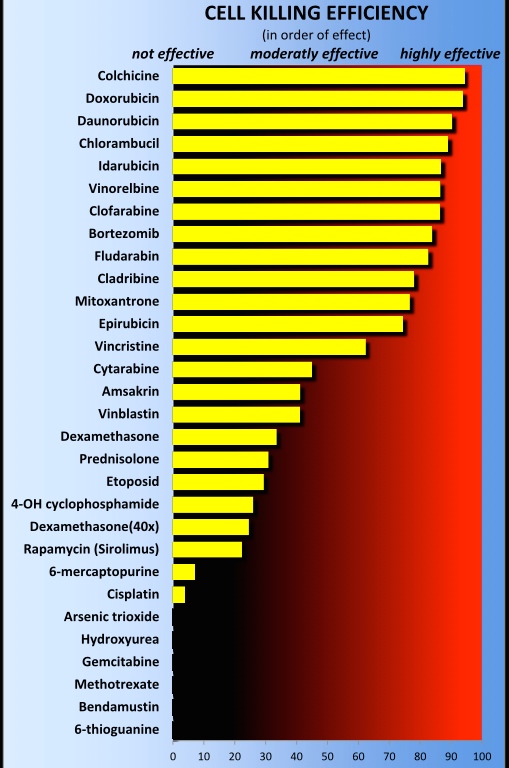
Cell Killing Efficiency bars are representing the different drugs on the iVV Assay™ plate, ranked according to which drug kills the patient tumor cells most effectively.
The drugs are presented in 4 different concentrations where the highest concentration is the routinely used clinical dose. The lowest concentration is the 125 times dilution of the clinical dose. That drug, which can eliminate at least 50% of the tumor cells already at this very low concentration is called “highly effective”.
The KE bars have an easy read out feature and show the drugs ranked as highest and therefore recommended as possible therapy.
DRUG RESPONSE TITRATIONS
Titration curves act as a perfect quality control feature and demonstrate the titration dependent cell death. If the curves are not following the concentration, error messages are generated as warning signals, stating the possible failure options. It insures the assay being very robust and trustworthy.
DRUG LIST
QantaScope iVV assay™ drug coated 384-well plates are produced with a barcode. The barcode stores all important information about the different drugs, their highest concentration and their location on the 384-well plate. Analysis software is reproducing the stored data and report is generated in accordance to that.
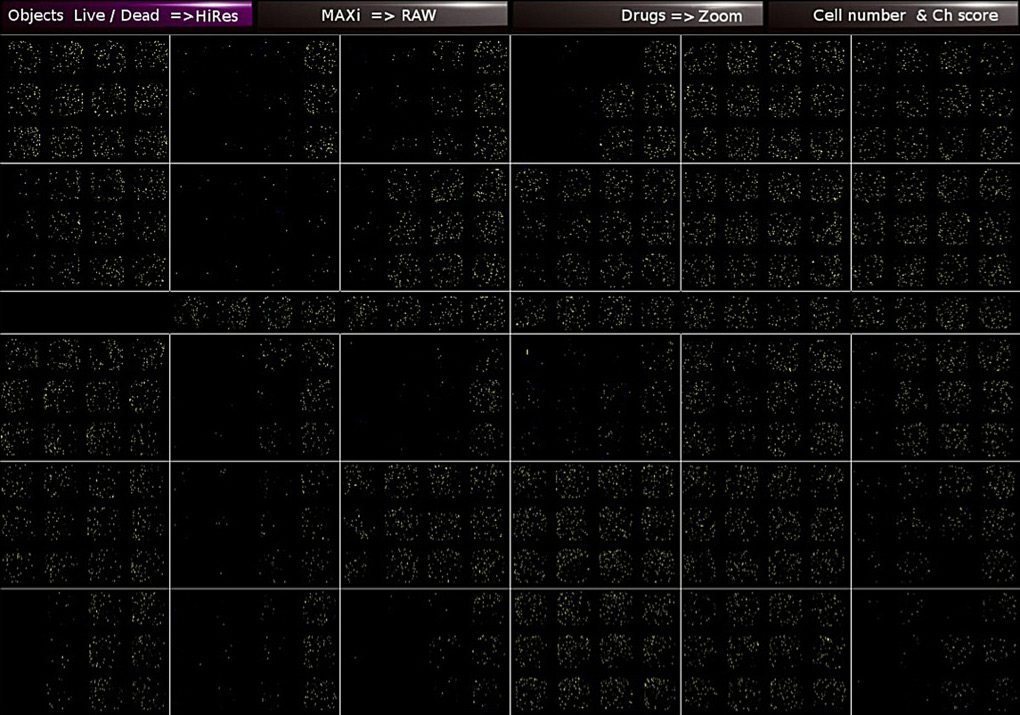
PLATE IMAGES
The user has a gateway to all raw capturing data, statistical data, mosaic pictures of the 384-well plates, picture of every single well, every single cell, and titration images for every single drug as well. This extra feature allows the user to evaluate the assay and the system. It is recommended to be used as quality check on a time by time basis
SOFTWARE FEATURES
During the scanning process, every cell is captured and analyzed for a number of image analytic parameters. The cells are counted using five different mathematical and statistical algorithms in triplicates to ensure the robustness of the system.
The instrument is controlled by a user-friendly software that permits uninterrupted batch mode capturing during working hours followed by automated analysis of the captured images. Analysis is made on a single cell level where counting of both living and dead cells are made automatically. Because of the robust analysis, both single cell cultures but also cells with medium level clumping properties can be measured.
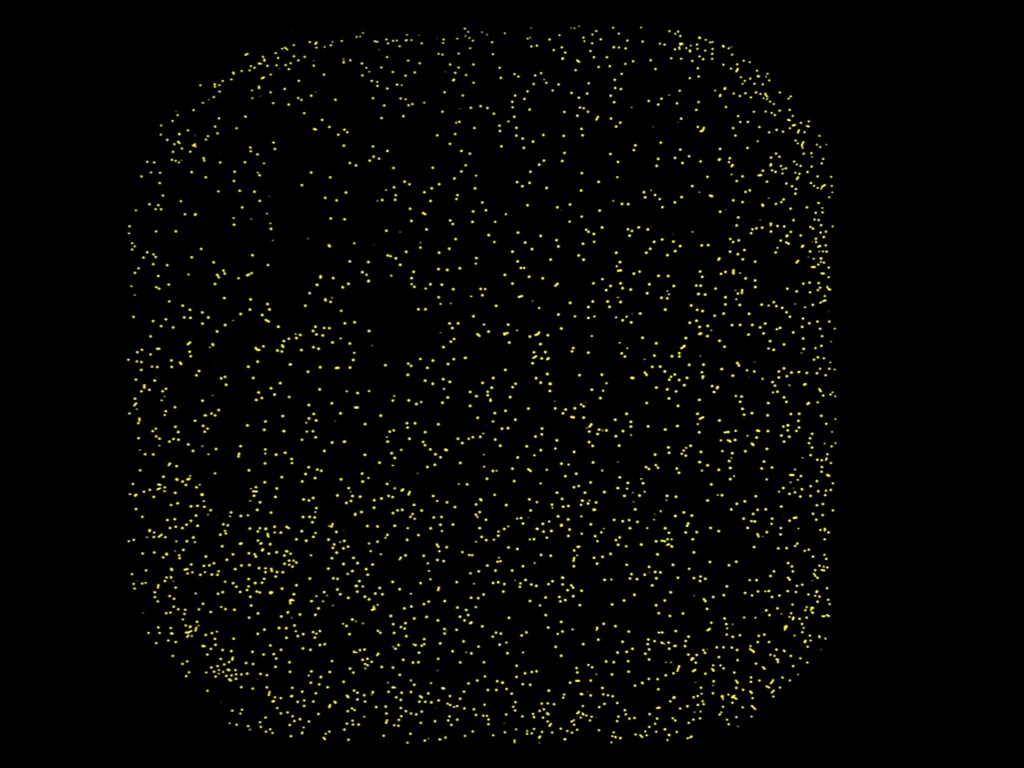
Live single cell image captured in one well on the 384-well plate.
Laboratory requirements
To be able to run iVV assay and Qantascope HexascopeHAEMA™ the following environmental and Good Laboratory Practice must be maintained.
- Sterile working technique
- Sterile hood for cell culturing
- 37℃ CO2 incubator
- refrigerator (+4℃-+8℃)
- freezer (-20℃)
The system consists of the scanning unit, the desktop computer with flat screen monitor and a barcode reader.
HexascopeHAEMA™ is for in vitro diagnostic use, CE and IVD certified. The instrument optimized to operate with QantaScope iVV assay™ reagent kits but can also be used for experimental drug screening or for patient preselection in clinical trials.
Instrument parameters
Scanning time: 9 minutes
Capacity: 22 plates/day with completely analyzed results
Presentation of results:
Killing efficiency ranking plot and individual live/dead titration curves for every drugs
Number of drugs measured: 30 drugs/plate/patient
Dimensions: W: 41cm L: 41cm H: 39cm
Weight: 25kg
Format: Desktop device
Scanning channels: 2 colors
Consists of: scanner unit, flat screen monitor, desktop computer, barcode reader, 1 quality control kit (Product code: QS-QC-BEADS-04)
CE and IVD certified.
Instrument and reagent productions are following the ISO9001:2008 directives.